|
|
 |
|
 |
|
|
|
|
 |
When Non-United States Citizens have adverse reactions in Clinical Trials |
 |
When United States Citizens have adverse reactions in Clinical Trials |
| The ABPI-BIA model Clinical Trial Agreement 2011 - England |
Link to U.S. agreement models for clinical trials. (DOES NOT EXIST) |
In Britain, patients who suffer adverse events are automatically treated and compensated per the above volutary agreement singed by Janssen and all other industry leaders. The Pharmaceutical companies are automatically protected from frivilous claims and do not pay for anything of a temporary or cureable nature.
|
This is how a US Senator refuses to help.
Thank you Senator. We took this route 5, 3 and 1 years ago.
Dear Lynanne:
Thank you for contacting me to share the story of Anthony Viscosi. I welcome this opportunity to respond.
I reviewed the information you referenced, which states that Mr. Viscosi began suffering from severe forms of psoriasis after participating in a clinical trial for the prescription medicine Stelera.
I believe it is vital that clinical trial researchers take action to protect subjects from any potentially negative side effects to the greatest extent possible and provide participants with information about the risks associated with participation. Food and Drug Administration (FDA) regulations require individuals to sign an "informed consent" form prior to participating in a clinical trial. This form must list possible risks and benefits of participating in the trial as well as information about whom to contact with questions, comments or in the case of injury. In addition, Institutional Review Boards (IRBs) must review clinical trials to ensure that risks to subjects are reasonable and have been minimized, patient information will be protected and that data collection methods are appropriate to safeguard patient safety.
Violations of federal regulations and other problems associated with clinical trials may be reported to the FDA Center for Drug Evaluation and Research. If you believe a clinical trial has violated patient safety standards, I encourage you to contact the FDA:
Center for Drug Evaluation and Research
Division of Scientific Investigation
Call 301-796-3150
Fax 301-847-8748
Email: DSI@fda.hhs.gov
I appreciate you reaching out to my office regarding this matter. I support stringent safety and informed consent standards for clinical trials and will be sure to keep your views in mind should related legislation come before the full Senate for consideration. If you have additional questions or comments, please don't hesitate to contact my Washington, D.C. office at (202) 224-3841. Best regards.
Sincerely yours,
Dianne Feinstein
United States Senator
You may also visit my website for more information regarding my position on health care issues. You can also follow me on Facebook, YouTube, and Twitter.
Questions about health reform? Click here to learn about new health insurance options and how you may benefit under the health reform law.
|
In Britain, when a patient in a clinical trial held by Janssen pharmaceutical has an adverse event, Janssen immediately and voluntarily jumps in and covers all of the patient's medical and personal needs.
They start a no nonsense investigation as to whether the event was caused by the clinical trial, but either way, cover the patient's needs until the investigation is complete.
The investigation hinges on one main question. Did the patient have the symptoms of the adverse event prior to starting the trial? If the answer is no, then in most cases, the investigation is over.
If the adverse event proves serious and long lasting, Janssen's insurance automatically provides compensation up to $2.5 Million pounds. Period.
In the U.S.,when a patient in a clinical trial held by Janssen pharmaceutical has an adverse event, Janssen is under no obligation to even provide the patient with information about the drug that ruined their life, let alone provide compensation or medical expertise.
If the patient needs any sort of information, they must file a report with the FDA, wait up to six months, then file another report with Janssen and wait for the information.
If the patient needs any sort of compensation due to medical costs, loss of income or suffering, their only recourse is ask the FDA and the Center for Drug Evanluation and Research Division of Scientific Investigation, to start an investigation.
The patient must wait 180 days for an investigation to begin.
If they do choose to investigate, the patient must then prove somehow that the drug was the cause of the event.
If somehow they do prove the drug caused the event, it is 100% upon the sick or dying patient to source an attorney, convince them of the financial viability of a law suit, then wait as up to seven years to see if the suit is successful or settled.
Why the huge difference in how the same situation is handled in 2 countries who are considered "sister" countries?
In Britain, the British Healthcare Business Intelligence Association (BHBIA), British version of the AMA (American Medical Association) worked with elected representatives and the industry itself, and simply asked Janssen and all other industry leaders to sign a completely voluntary set of guidelines. It is a simple, straight forward 30 page document that governs all aspects of clinical trials. In fact, the portion that details how adverse events are handled is only 3 pages long.
This agreement was sponsored by and signed by each member of the (BHBIA), including Janssen Pharmaceutical, which makes Stelara.
In the U.S. Neither the AMA nor any elected government representative has, or will ask for the same dignity and safety for it's citizen patients as is universally done not only in Britain, but in most other countries of the world.
In particular, an adverse event that occurred in 2008 during a clinical trial of Janssen's now $1.4 Billion blockbuster drug Stelara
This site is dedicated to:
- Bringing attention to Tony, the Clinical Trials Victim.
- Bringing the standards of care for all clinical trials victims up to the standards of our British bretheren.
|
We are collecting information about Stelara and psoriatic worsening.
Please comment.
Tell us about your experiences with Stelara and other biologic drugs.
Why did you begin Stelara treatment?
Have you ever participated in a clinical trial?
Have you had an adverse event?
Was your psoriasis worse once you ended Stelara or other biologic treatment than before you began?
Do you think that Stelara made your psoriasis much worse once you could no longer take it?
Do you think that your psoriasis would have not worsened if you would have never taken Stelara?
Did you take Stelara for Psoriatic Arthritis?
Did your Psoriatic Arthritis improve using Stelara?
Did your Psoriatic Arthritis worsen using Stelara?
Did you have psoriasis prior to beginning Stelara treatment?
Did you have any “autoimmune” disorder before beginning Stelara treatment?
Did you develop any new autoimmune disorder during or after Stelara treatment?
Have you ever contacted the FDA about your Stelara treatment experience?
Did the FDA respond to your contact about your experience?
* More evidence is coming to light showing that Stelara, after clearing psoriasis, causes dramatic worsening of psoriasis, psoriatic arthritis and other autoimmune diseases. |
|
|
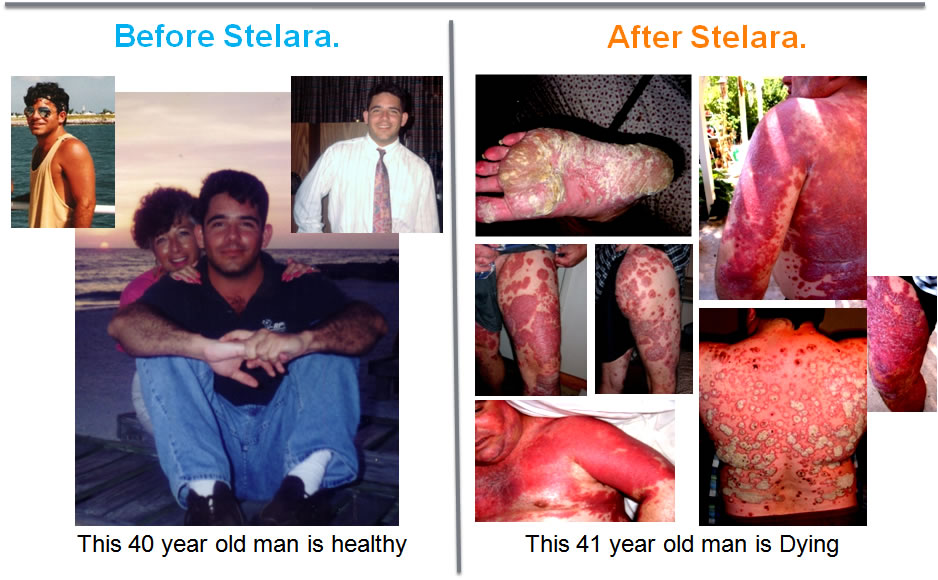
An "FDA APPROVED" drug did this to a healthy man.
A man who had only mild Psoriasis for over 7 years.
He then took part in a Clinical Trial of Stelara to treat his mild psoriasis.
|
|
|
|
as a printable document
|
|
SME Statements
| 'One mistake was to give the most powerful psoriatic drug ever made to someone who had very mild psoriasis' - PK, PMP |
'The Doctors running this trial certified that this patient had no other types of psoriasis by allowing him in the trial at all. A Documented Exclusion for this Clinical Trial: "Any non-plaque psoriasis" is that certification. If any other psoriasis was present, the Doctors would have disqualified him.' - EV, PI |
'Without specific clinical data, that only the drug's developers can provide, there is little hope that an effective treatment plan can be developed' - BO, MD |
|
| |
|
(The senate uses web-forms with fields) Use “Health” or “Other” in the subject field”)
Support is appreciated in your own words, or a suggested wording is below, which you can copy and paste.
|
“Dear Senator,
I am a voter in your state. I am very troubled by the situation described at http://www.helpfortony.org/Anthony_Viscosi.pdf. I expect you will take the time to look into this situation.
This man is dying because of the negligence and irresponsibility of Johnson & Johnson following a clinical trial for Stelara, in which Mr. Viscosi participated. Neither Johnson & Johnson nor the FDA has offered any help whatsoever to this man as his health deteriorates.
Please contact Johnson & Johnson’s CEO, Alex Gorsky at (732) 524-0400 and express your expectation that Johnson & Johnson will make good its written promise to Mr. Viscosi (as presented when he entered its clinical trial program) to fully support him in case of adverse event, and will also follow the Johnson & Johnson corporate Credo, Paragraph 4: “Research must be carried on, innovative programs developed and mistakes paid for.” The Credo was personally written by the leader of the company in 1943; Mr. Gorsky spent a full 30 minutes orating in detail about the Credo at his annual shareholder meeting on April 26th, 2012.
Please contact Anthony Viscosi personally at helpfortony@helpfortony.org to express your support.
A catastrophic mistake was made with Mr Viscosi. It needs to be remedied. Thank you for your service.
Sincerely, Voter"
|
|
A shower with Tony. |
| |
|
|
|
|


